Abstract
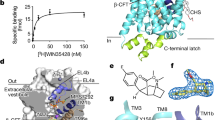
Antidepressants targeting Na+/Cl−-coupled neurotransmitter uptake define a key therapeutic strategy to treat clinical depression and neuropathic pain. However, identifying the molecular interactions that underlie the pharmacological activity of these transport inhibitors, and thus the mechanism by which the inhibitors lead to increased synaptic neurotransmitter levels, has proven elusive. Here we present the crystal structure of the Drosophila melanogaster dopamine transporter at 3.0 Å resolution bound to the tricyclic antidepressant nortriptyline. The transporter is locked in an outward-open conformation with nortriptyline wedged between transmembrane helices 1, 3, 6 and 8, blocking the transporter from binding substrate and from isomerizing to an inward-facing conformation. Although the overall structure of the dopamine transporter is similar to that of its prokaryotic relative LeuT, there are multiple distinctions, including a kink in transmembrane helix 12 halfway across the membrane bilayer, a latch-like carboxy-terminal helix that caps the cytoplasmic gate, and a cholesterol molecule wedged within a groove formed by transmembrane helices 1a, 5 and 7. Taken together, the dopamine transporter structure reveals the molecular basis for antidepressant action on sodium-coupled neurotransmitter symporters and elucidates critical elements of eukaryotic transporter structure and modulation by lipids, thus expanding our understanding of the mechanism and regulation of neurotransmitter uptake at chemical synapses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jessell, T. M. & Kandel, E. R. Synaptic transmission: a bidirectional and self-modifiable form of cell-cell communication. Cell 72 (Suppl). 1–30 (1993)
Masson, J., Sagne, C., Hamon, M. & El Mestikawy, S. Neurotransmitter transporters in the central nervous system. Pharmacol. Rev. 51, 439–464 (1999)
Kristensen, A. S. et al. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol. Rev. 63, 585–640 (2011)
Rudnick, G. Ion-coupled neurotransmitter transport: thermodynamic vs. kinetic determinations of stoichiometry. Methods Enzymol. 296, 233–247 (1998)
Radian, R., Bendahan, A. & Kanner, B. I. Purification and identification of the functional sodium- and chloride-coupled γ-aminobutyric acid transport glycoprotein from rat brain. J. Biol. Chem. 261, 15437–15441 (1986)
Waldman, I. D. et al. Association and linkage of the dopamine transporter gene and attention-deficit hyperactivity disorder in children: heterogeneity owing to diagnostic subtype and severity. Am. J. Hum. Genet. 63, 1767–1776 (1998)
Shannon, J. R. et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N. Engl. J. Med. 342, 541–549 (2000)
Meldrum, B. S. Neurotransmission in epilepsy. Epilepsia 36 (suppl. 1). 30–35 (1995)
Kurian, M. A. et al. Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile parkinsonism-dystonia. J. Clin. Invest. 119, 1595–1603 (2009)
Kuhn, R. The treatment of depressive states with G 22355 (imipramine hydrochloride). Am. J. Psychiatry 115, 459–464 (1958)
Axelrod, J., Whitby, L. G. & Hertting, G. Effect of psychotropic drugs on the uptake of H3-norepinephrine by tissues. Science 133, 383–384 (1961)
Berton, O. & Nestler, E. J. New approaches to antidepressant drug discovery: beyond monoamines. Nature Rev. Neurosci. 7, 137–151 (2006)
Pletscher, A. The discovery of antidepressants: a winding path. Experientia 47, 4–8 (1991)
Anderson, I. M. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. J. Affect. Disord. 58, 19–36 (2000)
Yamashita, A., Singh, S. K., Kawate, T., Jin, Y. & Gouaux, E. Crystal structure of a bacterial homologue of Na+/Cl–-dependent neurotransmitter transporters. Nature 437, 215–223 (2005)
Singh, S. K., Piscitelli, C. L., Yamashita, A. & Gouaux, E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science 322, 1655–1661 (2008)
Krishnamurthy, H. & Gouaux, E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature 481, 469–474 (2012)
Beuming, T. et al. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nature Neurosci. 11, 780–789 (2008)
Sørensen, L. et al. Interaction of antidepressants with the serotonin and norepinephrine transporters: mutational studies of the S1 substrate binding pocket. J. Biol. Chem. 287, 43694–43707 (2012)
Pörzgen, P., Park, S. K., Hirsh, J., Sonders, M. S. & Amara, S. G. The antidepressant-sensitive dopamine transporter in Drosophila melanogaster: a primordial carrier for catecholamines. Mol. Pharmacol. 59, 83–95 (2001)
Serrano-Vega, M. J., Magnani, F., Shibata, Y. & Tate, C. G. Conformational thermostabilization of the β1-adrenergic receptor in a detergent-resistant form. Proc. Natl Acad. Sci. USA 105, 877–882 (2008)
Tatsumi, M., Groshan, K., Blakely, R. D. & Richelson, E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur. J. Pharmacol. 340, 249–258 (1997)
Torres, G. E. et al. Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J. Biol. Chem. 278, 2731–2739 (2003)
Sitte, H. H., Farhan, H. & Javitch, J. A. Sodium-dependent neurotransmitter transporters: oligomerization as a determinant of transporter function and trafficking. Mol. Interv. 4, 38–47 (2004)
Li, L. B. et al. The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J. Biol. Chem. 279, 21012–21020 (2004)
Chen, R. et al. Direct evidence that two cysteines in the dopamine transporter form a disulfide bond. Mol. Cell. Biochem. 298, 41–48 (2007)
Norregaard, L., Frederiksen, D., Nielsen, E. O. & Gether, U. Delineation of an endogenous zinc-binding site in the human dopamine transporter. EMBO J. 17, 4266–4273 (1998)
Buck, K. J. & Amara, S. G. Structural domains of catecholamine transporter chimeras involved in selective inhibition by antidepressants and psychomotor stimulants. Mol. Pharmacol. 48, 1030–1037 (1995)
Chen, J. G., Sachpatzidis, A. & Rudnick, G. The third transmembrane domain of the serotonin transporter contains residues associated with substrate and cocaine binding. J. Biol. Chem. 272, 28321–28327 (1997)
Henry, L. K. et al. Tyr-95 and Ile-172 in transmembrane segments 1 and 3 of human serotonin transporters interact to establish high affinity recognition of antidepressants. J. Biol. Chem. 281, 2012–2023 (2006)
Bismuth, Y., Kavanaugh, M. P. & Kanner, B. I. Tyrosine 140 of the γ-aminobutyric acid transporter GAT-1 plays a critical role in neurotransmitter recognition. J. Biol. Chem. 272, 16096–16102 (1997)
Kitayama, S. et al. Dopamine transporter site-directed mutations differentially alter substrate transport and cocaine binding. Proc. Natl Acad. Sci. USA 89, 7782–7785 (1992)
Andersen, J. et al. Location of the antidepressant binding site in the serotonin transporter: importance of Ser-438 in recognition of citalopram and tricyclic antidepressants. J. Biol. Chem. 284, 10276–10284 (2009)
Talvenheimo, J., Fishkes, H., Nelson, P. J. & Rudnick, G. The serotonin transporter-imipramine “receptor”. J. Biol. Chem. 258, 6115–6119 (1983)
Singh, S. K., Yamashita, A. & Gouaux, E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature 448, 952–956 (2007)
Zhou, Z. et al. LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science 317, 1390–1393 (2007)
Zhou, Z. et al. Antidepressant specificity of serotonin transporter suggested by three LeuT–SSRI structures. Nature Struct. Mol. Biol. 16, 652–657 (2009)
Harding, M. M. Metal–ligand geometry relevant to proteins and in proteins: sodium and potassium. Acta Crystallogr. D 58, 872–874 (2002)
Forrest, L. R., Tavoulari, S., Zhang, Y. W., Rudnick, G. & Honig, B. Identification of a chloride ion binding site in Na+/Cl−-dependent transporters. Proc. Natl Acad. Sci. USA 104, 12761–12766 (2007)
Zomot, E. et al. Mechanism of chloride interaction with neurotransmitter:sodium symporters. Nature 449, 726–730 (2007)
Kantcheva, A. K. et al. Chloride binding site of neurotransmitter sodium symporters. Proc. Natl Acad. Sci. USA 110, 8489–8494 (2013)
Tavoulari, S., Forrest, L. R. & Rudnick, G. Fluoxetine (Prozac) binding to serotonin transporter is modulated by chloride and conformational changes. J. Neurosci. 29, 9635–9643 (2009)
Scanlon, S. M., Williams, D. C. & Schloss, P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry 40, 10507–10513 (2001)
North, P. & Fleischer, S. Alteration of synaptic membrane cholesterol/phospholipid ratio using a lipid transfer protein. Effect on γ-aminobutyric acid uptake. J. Biol. Chem. 258, 1242–1253 (1983)
Hong, W. C. & Amara, S. G. Membrane cholesterol modulates the outward facing conformation of the dopamine transporter and alters cocaine binding. J. Biol. Chem. 285, 32616–32626 (2010)
Bennett, E. R., Su, H. & Kanner, B. I. Mutation of arginine 44 of GAT-1, a (Na+ + Cl−-coupled γ-aminobutyric acid transporter from rat brain, impairs net flux but not exchange. J. Biol. Chem. 275, 34106–34113 (2000)
Cao, Y., Li, M., Mager, S. & Lester, H. A. Amino acid residues that control pH modulation of transport-associated current in mammalian serotonin transporters. J. Neurosci. 18, 7739–7749 (1998)
Loland, C. J., Norregaard, L., Litman, T. & Gether, U. Generation of an activating Zn2+ switch in the dopamine transporter: mutation of an intracellular tyrosine constitutively alters the conformational equilibrium of the transport cycle. Proc. Natl Acad. Sci. USA 99, 1683–1688 (2002)
Holton, K. L., Loder, M. K. & Melikian, H. E. Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization. Nature Neurosci. 8, 881–888 (2005)
Kawate, T. & Gouaux, E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 14, 673–681 (2006)
Dukkipati, A., Park, H. H., Waghray, D., Fischer, S. & Garcia, K. C. BacMam system for high-level expression of recombinant soluble and membrane glycoproteins for structural studies. Protein Expr. Purif. 62, 160–170 (2008)
Reeves, P. J., Callewaert, N., Contreras, R. & Khorana, H. G. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl Acad. Sci. USA 99, 13419–13424 (2002)
Baconguis, I. & Gouaux, E. Structural plasticity and dynamic selectivity of acid-sensing ion channel-spider toxin complexes. Nature 489, 400–405 (2012)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D 68, 352–367 (2012)
Terwilliger, T. C. et al. Iterative model building, structure refinement and density modification with the PHENIX AutoBuild wizard. Acta Crystallogr. D 64, 61–69 (2008)
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010)
Quick, M. & Javitch, J. A. Monitoring the function of membrane transport proteins in detergent-solubilized form. Proc. Natl Acad. Sci. USA 104, 3603–3608 (2007)
Giros, B. et al. Cloning, pharmacological characterization, and chromosome assignment of the human dopamine transporter. Mol. Pharmacol. 42, 383–390 (1992)
Acknowledgements
We thank D. Cawley for generating monoclonal antibodies and S. Amara for providing the wild-type Drosophila DAT construct. We would like to thank H. Wang and D. Claxton for comments and suggestions along with other Gouaux laboratory members for discussions during manuscript preparation. We thank L. Vaskalis for assistance with figures and H. Owen for help with manuscript preparation. We thank the staff of the Northeastern Collaborative Access Team (NECAT) at the Advanced Photon Source (APS) for assistance with data collection. This work was supported by a postdoctoral fellowship from the American Heart Association (A.P.), a National Institute of Mental Health research award (K.H.W.) and by the National Institutes of Health (E.G.) E.G. is an investigator with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
A.P., K.H.W. and E.G. designed the project. A.P. and K.H.W. performed protein purification, crystallography and biochemical assays. A.P., K.H.W. and E.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-3 and Supplementary Figures 1-8. (PDF 9370 kb)
Rights and permissions
About this article
Cite this article
Penmatsa, A., Wang, K. & Gouaux, E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 503, 85–90 (2013). https://doi.org/10.1038/nature12533
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12533
This article is cited by
-
GABA transport cycle: beyond a GAT feeling
Nature Structural & Molecular Biology (2023)
-
Structure and mechanism of the SGLT family of glucose transporters
Nature (2022)
-
The dopamine transporter antiports potassium to increase the uptake of dopamine
Nature Communications (2022)
-
Reconstitution of GABA, Glycine and Glutamate Transporters
Neurochemical Research (2022)
-
Serotonin Transporter Ala276 Mouse: Novel Model to Assess the Neurochemical and Behavioral Impact of Thr276 Phosphorylation In Vivo
Neurochemical Research (2022)