Abstract
This study investigated the discriminatory features of severe acute respiratory syndrome (SARS) and severe non-SARS community-acquired viral respiratory infection (requiring hospitalization) in an emergency department in Hong Kong. In a case-control study, clinical, laboratory and radiological data from 322 patients with laboratory-confirmed SARS from the 2003 SARS outbreak were compared with the data of 253 non-SARS adult patients with confirmed viral respiratory tract infection from 2004 in order to identify discriminatory features. Among the non-SARS patients, 235 (93%) were diagnosed as having influenza infections (primarily H3N2 subtype) and 77 (30%) had radiological evidence of pneumonia. In the early phase of the illness and after adjusting for baseline characteristics, SARS patients were less likely to have lower respiratory symptoms (e.g. sputum production, shortness of breath, chest pain) and more likely to have myalgia (p < 0.001). SARS patients had lower mean leukocyte and neutrophil counts (p < 0.0001) and more commonly had “ground-glass” radiological changes with no pleural effusion. Despite having a younger average age, SARS patients had a more aggressive respiratory course requiring admission to the ICU and a higher mortality rate. The area under the receiver operator characteristic curve for predicting SARS when all variables were considered was 0.983. Using a cutoff score of >99, the sensitivity was 89.1% (95%CI 82.0–94.0) and the specificity was 98.0% (95%CI 95.4–99.3). The area under the receiver operator characteristic curve for predicting SARS when all variables except radiological change were considered was 0.933. Using a cutoff score of >8, the sensitivity was 80.7% (95%CI 72.4–87.3) and the specificity was 94.5% (95%CI 90.9–96.9). Certain clinical manifestations and laboratory changes may help to distinguish SARS from other influenza-like illnesses. Scoring systems may help identify patients who should receive more specific tests for influenza or SARS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe acute respiratory syndrome (SARS) is a novel, infectious, respiratory disease caused by a SARS-coronavirus (SARS-CoV) [1–3] that manifested in a global outbreak in 2003 [4]. Over 99% of SARS cases are characterized by pneumonia [5], and in the 2003 SARS outbreak an estimated 1,755 patients in Hong Kong were affected with an overall mortality of 17%. SARS re-emerges sporadically, as evidenced by the high prevalence of antibodies against SARS-CoV in animal traders in Guangdong [6], and thus poses a continuing threat. During the 2003 outbreak, the diagnostic accuracy of clinicians in Hong Kong was high, facilitated by the absence of any coinciding influenza or other respiratory infections [4]. However, the clinical, radiological and laboratory features of SARS are non-specific [7–10]. Therefore, if a coexisting outbreak of acquired pneumonia had occurred in the SARS-CoV-negative community, the diagnosis would have been hampered, with potentially catastrophic results.
The non-specific nature of many of the symptoms associated with SARS makes the development of sensitive diagnostic tests challenging, particularly if a SARS reemergence were to be concurrent with an epidemic of another respiratory disease, such as influenza. Although initial case definitions were insensitive for diagnosing SARS early in the course of the illness [11], and prediction guidelines have subsequently been developed to help diagnose and stratify SARS [12, 13], these guidelines have never been tested during a co-existent outbreak of influenza, bacterial infection or any other common cause of community-acquired pneumonia.
Worldwide, influenza is estimated to be responsible for 4.1–4.5 million excess illnesses annually in individuals aged 20 years and older [14]. The symptoms of this disease are debilitating. Influenza-related complications, such as lower respiratory illnesses (e.g. bronchitis, pneumonia) and other systemic complications (e.g. cardiovascular, cerebrovascular diseases) commonly result in approximately 150,000 hospitalizations and 20,000 deaths annually in the USA alone [14].
The aim of this study was to compare early clinical, radiological and laboratory results collected during the SARS outbreak of 2003 with those collected during non-SARS outbreaks of viral respiratory illnesses in 2004 for patients presenting from the same community to the same hospital. If early discriminating features are recognized, they may facilitate the diagnosis of SARS and hence the timely control of SARS transmission, since the overall detection rate for SARS-CoV RNA by PCR assays is currently only 60% in the first week of illness [15, 16]. It has been postulated that improvement of diagnostic guidelines specific to SARS would be much more cost-effective than testing with PCR assays; establishing an alternative diagnosis by influenza testing is another cost-effective strategy [17]. The World Health Organization has continued to call for the improvement of diagnostic guidelines for distinguishing SARS from other respiratory illnesses [18].
Patients and methods
Study design and setting
This case-control study was conducted at the emergency department of the Prince of Wales Hospital in Hong Kong. The SARS crisis in 2003 was so rapid and severe it was not possible to pursue normal channels of approval and consent for the duration of the outbreak. Nevertheless, all patients were given a questionnaire to answer and were informed that the data would be used for surveillance, analysis and research. The chief executives and associated institutional review boards of the Hospital Authority of Hong Kong and of the Prince of Wales Hospital later approved the collection of clinical data and the use of blood tests for surveillance, analysis, research and reporting. Many of the SARS patients included in this study have been described in previous reports [4, 7, 11, 12]. Approval was obtained from the institutional review boards for the second phase of the study, which involved the prospective collection of data from patients with severe viral respiratory illnesses admitted to hospital in 2004.
The records of 322 patients with laboratory-confirmed SARS who were admitted to Prince of Wales Hospital during the SARS outbreak of 2003 between February and June were reviewed. For comparison, data was collected prospectively from 253 consecutive non-SARS patients with febrile respiratory illness and likely lower respiratory tract involvement; these patients were diagnosed with other viral etiologies and were admitted to hospital through the emergency department between 1 January and 31 December 2004.
The Prince of Wales Hospital is a 1,400-bed teaching hospital and tertiary referral centre for the New Territories of the Hong Kong Special Administrative Region. It serves a mainly urban catchment area of 1.5 million people. The emergency department sees 160,000 new patients per annum, and admits 24% of them. Approximately 50 patients are admitted to internal medicine wards each day, and 20% of these admissions are due to infective causes.
Inclusion and exclusion criteria
Patients were included in the study if they met the following criteria: (a) adult patients aged ≥18 years; (b) presented to the emergency department and required hospitalization; (c) had a febrile respiratory illness, as defined by the presence of lower respiratory symptoms (cough, sputum production, shortness of breath), with or without radiological features of pneumonia (haziness or definite pneumonia) [19]; and (d) had positive virological confirmation. Patients without established viral etiologies were excluded.
Patient assessments and investigations
Patients were seen by an emergency specialist and a physician. Patients completed a health questionnaire that documented contact history, as well as upper and lower respiratory tract, gastrointestinal and systemic symptoms. Pulse rate, systolic and diastolic blood pressure, respiratory rate, tympanic temperature and oxygen saturation at room air were also recorded as observations at presentation. All patients received frontal plain chest radiographs, which were initially evaluated by a specialist emergency physician with reference to clinical details, and then by a radiologist blinded to clinical information. A clear contact history was established if a person had close physical contact with another person known to have a similar infection.
Virological investigations
The laboratory confirmation of SARS-CoV was performed as described previously [20, 21] according to World Health Organization criteria. Briefly, SARS was confirmed if any one of three criteria were met: (a) a fourfold rise in SARS-CoV antibody titer between acute- (taken within 7 days after the onset of fever) and convalescent- (taken at least 3 weeks after the onset of fever) phase serum; (b) detection of antibody to SARS-CoV in specimens during acute illness; or (c) detection of antibody to SARS-CoV in specimens obtained from convalescent serum. Serologically negative cases were defined as those in which antibody to SARS-CoV was absent in convalescent serum taken ≥28 days after symptom onset [22].
For other respiratory viruses, a combination of viral antigen detection by the immunofluorescence test, virus isolation and serology were used. Nasopharyngeal aspirates were collected for rapid viral antigen detection by immunofluorescence for influenza A and B, and respiratory syncytial virus using commercial assays (Dako Diagnostic). These aspirates were collected either in the emergency department or within the first few hours after admission to a ward. Results were generally available on the same working day. Virus isolation was performed using rhesus monkey kidney (LLC-MK2), human laryngeal carcinoma (HEp-2), Mardin Darby Canine Kidney (MDCK), human embryonic lung fibroblast, Buffalo green monkey kidney (BGM), and African green monkey kidney (Vero) monolayers. Cell monolayers were examined daily for cytopathic effect. After 14 days of incubation, the growth of influenza A and B, parainfluenza types 1, 2, and 3, respiratory syncytial virus (RSV) and adenovirus were examined using commercial monoclonal antibodies with the immunofluorescence technique. In addition, the hemadsorption test was performed for LLC-MK2 and MDCK monolayers. Paired sera were subjected to antibody detection by complement fixation tests covering influenza A and B, respiratory syncytial virus, parainfluenza viruses 1, 2 and 3, adenovirus and Mycoplasma pneumoniae.
Sputum specimens were also sent for Gram stain and bacterial culture, Ziehl–Neelsen stain and mycobacterial culture. Peripheral blood was sent for culture. Urine was sent for rapid antigen testing for Pneumococcus spp and Legionella pneumophila serogroup 1 whenever clinically indicated.
Chest radiography
Imaging technique
Due to the high infectivity of the disease and the volume of patients seen during the SARS outbreak, only frontal radiographs were performed for most patients, and these were used for analysis in this study. As a precaution, all inpatient chest radiographs were performed with mobile units. Film handling was minimized and, for this project only, digital images were reviewed. Chest radiographs were performed following an established standard protocol. Frontal chest radiographs were obtained, in the postero-anterior projection for patients who could stand, or anteroposterior views for those who could not. A similar approach was used for the non-SARS patients in this study. All radiological examinations were performed using radiological equipment (Mobilett Plus; Siemens, Erlangen, Germany) and a standardized technique: 75 kV, 4 mAs, and a 180-cm film-focus distance for the posteroanterior views and 70 kV, 4 mAs, and a 100-cm film-focus distance for the anteroposterior views; a broad tube focus was used to obtain both views.
Radiograph evaluation
The images were assessed using a picture archiving and communication system, or PACS, viewer workstation with a 2,048 × 2,048-pixel monitor (Magicview, version VA22E; Siemens). The images were reviewed by radiologists working in pairs and the interpretation was by consensus.
Classification/scoring system
Each lung was divided into upper, middle, and lower zones. Each of the three zones encompassed one-third of the craniocaudal distance of the lung on a frontal radiograph and was evaluated separately. The radiologists assessed and recorded the presence, appearance (airspace opacity, reticular opacity, mass, nodule, pleural effusion, and/or lymphadenopathy), and distribution (upper, middle, or lower lung zone involvement and unilateral or bilateral involvement) of changes observed on the frontal radiograph.
Statistical analysis
Demographic, clinical, laboratory and radiological features were analyzed using the unpaired Student’s t test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. Data were analyzed using Statview® for Windows version 7.1 statistical analysis software (Abacus Concepts, SAS Institute, Cary NC, USA). Data is presented in terms of means and standard deviations unless otherwise specified. All analyses were two tailed and a p-value of <0.05 was considered statistically significant.
In the first instance, there were too many variables to construct a single multivariate logistic regression model. Therefore, adjusted odds ratios were calculated by constructing four separate logistic regression models with different sets of variables – clinical, observations at presentation, laboratory and radiological. Age and sex were initially entered into each of these models. For each model, variables that failed to meet the significance levels of the likelihood ratio test (p > 0.05) were removed by backward stepwise elimination until all insignificant variables had been removed. The remaining significant variables from each of the four initial models were then entered into a final multivariate logistic regression model. Insignificant variables were again removed by backward stepwise elimination. Scores were assigned to each of the remaining variables based on their logistic odds ratios. Receiver operator characteristic (ROC) curves for sensitivity and specificity were constructed based on these scores.
Results
During the 2003 SARS outbreak, 322 of 1,530 (21%) patients screened at Prince of Wales Hospital were confirmed to have SARS-CoV infection. During 2004, 1,461 patients were admitted from the emergency department to a medical ward with a provisional diagnosis of febrile respiratory illness with or without pneumonia; among them, 253 (17%) had SARS-CoV-negative respiratory tract infection confirmed to be of other viral etiologies.
Compared with SARS-CoV-negative patients, patients with SARS were younger (41.3 ± 18.4 vs 67.8 ± 19.4 years; p < 0.01), more likely to be healthcare workers (176 [55%] vs 0; p < 0.01), and more likely to have a clear contact history (271 [84%] vs 1; p < 0.01). Influenza A accounted for 216 (85%) patients with viral respiratory tract infection, influenza B for 19 (8%) patients, and respiratory syncytial virus for 12 (5%) patients admitted in 2004. Among the cases in which subtyping data was available, 61 of 253 (24%) infections were due to subtypes H3N2, and 10 (4%) were due to H1N1.
Table 1 shows the clinical features and observations at presentation of the 575 patients who presented within a mean time from symptom onset of 4 days (SD 3). The following features were more common in non-SARS than SARS cases in univariate analysis: cough, runny nose, sputum production, chest pain, shortness of breath, vomiting, and impaired general condition (elderly). Chills, malaise, myalgia, rigors, diarrhea and headache were more common in SARS than non-SARS cases. Patients from the non-SARS group had a higher mean systolic blood pressure, respiratory rate and initial temperature reading and lower mean oxygen saturation on room air than those in the SARS group.
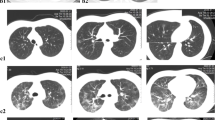
Although most patients were admitted to the ward based on the emergency physicians’ impression that they had a febrile respiratory illness with possible lower respiratory tract involvement, only 77 (30%) patients in 2004, and 305 (95%) SARS cases in 2003 had definite pneumonia visible on radiographs (Table 2). Most (98%) of the patients with SARS pneumonia had ground-glass opacification on chest radiograph; in comparison, the non-SARS pneumonia patients demonstrated ground-glass opacification in 17% of cases. Pleural effusion was not a feature of SARS viral infection, but it was present in 14 (7%) cases of non-SARS viral infection.
Patients were more likely to be admitted quickly at the first emergency department presentation during 2004 than during the SARS outbreak of 2003 (Table 3). SARS patients were more likely to require admission to the ICU than patients with non-SARS viral respiratory tract infection. Six patients presenting early in the course of illness when no diagnostic tests were available were not admitted to the ward. Subsequent testing identified these as SARS cases. The overall mortality of our SARS patients (10%) was greater than that of patients with non-SARS viral infection (3%) and was similar to the global mortality of 9.6% for SARS [23].
The mean ages of survivors and patients who died after non-SARS viral infection were 68 (SD 19) and 61 (SD 32) years, respectively, (difference –7, p = 0.31), whereas the mean ages of survivors and patients who died after SARS viral infection were 38 (SD 15) and 73 (SD 13) years, respectively, (difference 35, p < 0.01). After adjustments were made for age and sex, the odds ratio for death in patients with SARS versus non-SARS viral infection was 24.39 (95%CI 9.52–62.50, p < 0.01). Age-specific subgroup analysis is shown in Table 4. For patients over 50 years of age the mortality rates after SARS infection were much higher than those for non-SARS patients.
Initially, four separate models were constructed – clinical, observations at presentation, laboratory and radiological. After adjusting for age and sex, the clinical features most discriminatory were myalgia, sputum production, runny nose, chest pain and shortness of breath, each of which should be considered as an independent discriminating factor (Table 5). The most discriminatory observations and laboratory findings were respiratory rate, temperature, total leukocyte count, neutrophil count and lymphocyte count, all of which should be considered as discriminating factors. The presence of a ground-glass appearance on chest radiograph was the strongest discriminating factor.
Variables from Table 5 were combined into a final model and insignificant variables were removed by backward stepwise elimination (Table 6). The significant variables that remained in favor of SARS infection were age ≤60 years, myalgia, respiratory rate ≤20 breaths per minute, temperature ≤38°C, leukocyte count ≤7.0 × 109/l, and ground-glass appearance on chest radiograph. The single significant factor against SARS infection was sputum production. Each of these variables was scored according to its odds ratio (Table 6).
The area under the ROC curve for predicting SARS when all variables were considered was 0.983. Using a cutoff score of >99, the sensitivity was 89.1% (95%CI 82.0–94.0) and the specificity was 98.0% (95%CI 95.4–99.3) (Fig. 1).
The area under the ROC curve for predicting SARS when all variables except radiographic change were considered was 0.933. Using a cutoff score of >8, the sensitivity was 80.7% (95%CI 72.4–87.3) and the specificity was 94.5% (95%CI 90.9–96.9) (Fig. 2).
Discussion
This study shows that the early clinical and laboratory presentation of patients with SARS differed significantly from that of patients with other common causes of viral respiratory tract infection and pneumonia. Although fever was common in cases of both SARS and non-SARS viral pneumonia, other systemic symptoms such as chills, malaise, and rigors were less common in non-SARS viral infections. Upper and lower respiratory symptoms were more common in non-SARS cases. Patients with non-SARS viral infections had higher systolic blood pressures and respiratory rates, and lower oxygen saturation readings than patients with SARS, which may reflect older age and premorbid status rather than pneumonia itself. The clinical diagnosis of pneumonia was supported by significant radiological changes more commonly in patients with SARS than non-SARS infection. Total leukocyte and neutrophil counts were higher in non-SARS viral pneumonia, but mean lymphocyte counts, although depressed, did not help discriminate among different etiologies. A recent study indicated that the absolute neutrophil count is discriminative between patients with SARS and those with other causes of community-acquired pneumonia [10]. In contrast, our study included only non-SARS viral etiologies. The routine total leukocyte and neutrophil counts may potentially be of use in excluding SARS and should be evaluated further.
SARS can be confused with influenza [1–5, 24, 25]. In fact, during work-up for any patient considered to be at high risk for SARS epidemiologically, the Centers for Disease Control and Prevention recommends routine testing for influenza A and B [22].
This is the first study to compare the clinical, laboratory and radiological features of SARS to those of influenza. It demonstrated that certain features are more commonly seen among SARS patients; if these are seen, SARS-CoV testing is probably indicated in the correct epidemiological setting [22]. On the other hand, specific SARS-CoV testing for all cases of acute febrile respiratory illness is not cost-effective and can cause social disruption due to the false-positive and false-negative results these tests can produce; this is especially true in the absence of worldwide transmission, since the prevalence of SARS is extremely low. Establishing an alternative diagnosis is a reasonable strategy and is likely to be more cost-effective [16]. This is especially important under the current threat of another pandemic flu. Although it is possible for a patient to have coincident SARS and influenza infections, the combination is unlikely. Nevertheless, during coincident SARS and influenza outbreaks, we cannot rule out the possibility that a patient may contract both.
Since patients in both groups of our study attended the emergency department within the first few days of illness, it is likely that the clinical and laboratory differences are not simply a result of measurements being taken at different times during the course of illness. Nevertheless, the present study had several limitations. First, it was conducted at a single centre, so the applicability of our findings may vary depending upon the relative prevalence of the etiological agent during any particular period. Second, we compared patients with known SARS or non-SARS viral infection, while this status is not typically known in presenting patients. Third, viral illness follows temporal patterns, so single data measurements obtained during the epidemic may not allow for temporal variation; however, our study did focus on a similarly early period in the course of the illness. Fourth, the radiological assessment of SARS cases in 2003 was not as thorough as that conducted for patients in 2004. During the SARS outbreak, the high infectivity of the disease and the large number of patients limited some of the assessments. Only frontal radiographs were performed for most patients, and many radiographs were performed with mobile units. In some cases, film handling was minimized and only digital images were reviewed. Nevertheless, chest radiographs and assessments were performed following an established standard protocol. Our non-SARS group may not be representative since they were hospitalized patients. Non-hospitalized influenza cases may resemble SARS even more closely.
The results of this study indicate SARS appears to have a more severe intermediate course than non-viral pneumonia and it accounts more frequently for admission to the ICU. The overall mortality was, nonetheless, the same for both groups, despite the higher mean age of patients in the non-SARS group. Although there are clinical, laboratory and radiological differences between SARS and commonly occurring non-SARS viral respiratory infections, specific influenza testing is still important since these conditions can mimic each other clinically, despite their differences. This is especially important given the threat of another influenza pandemic.
References
Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling AE, Humphrey CD, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ, SARS Working Group (2003) A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348:1953–1966
Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Nicholls J, Yee WK, Yan WW, Cheung MT, Cheng VC, Chan KH, Tsang DN, Yung RW, Ng TK, Yuen KY, SARS study group (2003) Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319–1325
Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguiere AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348:1967–1976
World Health Organization. Case definitions for surveillance of severe acute respiratory syndrome (SARS). http://www.who.int/csr/sars/casedefinition/en/. Cited 4 December 2003
Rainer TH, Chan PKS, Ip M, Lee N, Hui DS, Smit DeV, Wu A, Ahuja AT, Tam JS, Sung JY, Cameron P (2004) Preliminary analysis of the spectrum of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) infection in a large outbreak. Ann Intern Med 140:614–619
Morbidity and Mortality Weekly Report (2003) Prevalence of IgG antibody to SARS-associated coronavirus in animal traders Guangdong Province, China. MMWR 52:986–987
Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, Lui SF, Szeto CC, Chung S, Sung JJ (2003) A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 348:1986–1994
Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M (2003) A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med 348:1977–1985
Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, Walmsley SL, Mazzulli T, Avendano M, Derkach P, Ephtimios IE, Kitai I, Mederski BD, Shadowitz SB, Gold WL, Hawryluck LA, Rea E, Chenkin JS, Cescon DW, Poutanen SM, Detsky AS (2003) Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 289:2801–2809
Muller MP, Tomlinson G, Marrie TJ, Tsang P, McGeer A, Low DE, Detsky AS, Gold WL (2005) Can routine laboratory tests discriminate between severe acute respiratory syndrome and other causes of community-acquired pneumonia? Clin Infect Dis 40:1079–1086
Rainer TH, Cameron PA, Smit D, Ong KL, Hung AN, Nin DC, Ahuja AT, Si LC, Sung JJ (2003) Evaluation of WHO criteria for identifying patients with severe acute respiratory syndrome out of hospital: prospective observational study. BMJ 326:1354–1358
Leung G, Rainer TH, Lau FL, Wong IOL, Tong A, Wong T-W, Kong JHB, Hedley AJ, Lam T-H, for Hospital Authority SARS Collaborative Group (HASCOG) (2004) A clinical prediction rule to identify emergency room attendees with severe acute respiratory syndrome (SARS) during an outbreak. Ann Intern Med 141:333–342
Rainer TH, Wong IOL, Leung GM (2005) Severe acute respiratory syndrome. Ann Intern Med 142:225–226
Cox NJ, Subbarao K (1999) Influenza. Lancet 354:1277–1282
Centers for Disease Control and Prevention (2004) Public health guidance for community-level preparedness and response to severe acute respiratory syndrome (SARS) version 2/3. http://www.phppo.cdc.gov/PHTN/SARSIII/default.asp. Cited 5 October 2005
Chan KH, Poon LL, Cheng VC, Guan Y, Hung IF, Kong J, Yam LY, Seto WH, Yuen KY, Peiris JS (2004) Detection of SARS coronavirus (SCoV) by RT-PCR, culture, and serology in patients with acute respiratory syndrome (SARS). Emerg Infect Dis 10:294–299
Khan K, Muenning P, Gardam M, Zivin JG (2005) Managing febrile respiratory illnesses during a hypothetical SARS outbreak. Emerg Infect Dis 11:191–200
World Health Organization (2003) Summary of the discussion and recommendations of the SARS laboratory workshop. http://www.who.int/csr/sars/labmethods/en/. Cited 5 October 2005
American Thoracic Society (2001) Community-acquired pneumonia guidelines. Am J Respir Crit Care Med 163:1703
World Health Organization (2003) Use of laboratory methods for SARS diagnosis. http://www.who.int/csr/sars/labmethods/en/. Cited 4 December 2003
Chan PKS, To WK, Ng KC, Lam RKY, Ng TK, Chan RCW, Wu A, Yu WC, Lee N, Hui DSC, Lai ST, Hon EKL, Li CK, Sung JJY, Tam JS (2004). Laboratory diagnosis for severe acute respiratory syndrome. Emerg Infect Dis 10:825–831
Centers for Disease Control and Prevention (2003) Updated interim U.S. case definition for severe acute respiratory syndrome (SARS). http://www.cdc.gov/ncidod/sars. Cited 14 December 2006
Galvani AP, Lei X, Jewell NP (2003) Severe acute respiratory syndrome: temporal stability and geographic variation in case-fatality rates and doubling times. Emerg Infect Dis 9:991–994
Louie JK, Hacker JK, Mark J, Gavali SS, Yagi S, Espinosa A, Schnurr DP, Cossen CK, Isaacson ER, Glaser CA, Fischer M, Reingold AL, Vugia DJ, Unexplained deaths and critical illnesses working group (2004) Emerg Infect Dis 10:1143–1146
Kwan BC, Leung CB, Szeto CC, Wong VW, Cheng YL, Yu AW, Li PK (2003) Severe acute respiratory syndrome in a hemodialysis patient. Am J Kidney Dis 42:1069–1074
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rainer, T.H., Lee, N., Ip, M. et al. Features discriminating SARS from other severe viral respiratory tract infections. Eur J Clin Microbiol Infect Dis 26, 121–129 (2007). https://doi.org/10.1007/s10096-006-0246-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-006-0246-4