Abstract
Objectives
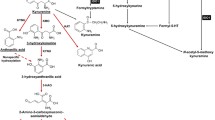
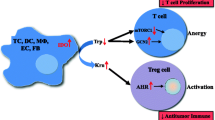
Indoleamine-2,3-Dioxygenase (IDO) is an immunosuppressive molecule inducible in various cells. In addition to classic IDO (IDO1), a new variant, IDO2, has recently been described. When expressed in dendritic cells (DCs) or cancer cells, IDO was thought to suppress the immune response to tumors. A novel therapeutic approach in cancer envisages inhibition of IDO with 1-methyl-tryptophan (1MT). The levo-isoform (l-1MT) blocks IDO1, whereas dextro-1MT (d-1MT), which is used in clinical trials, inhibits IDO2. Here we analyze IDO2 expression in human cancer cells and the impact of both 1-MT isoforms on IDO activity.
Methods
Surgically extirpated human primary tumors as well as human cancer cell lines were tested for IDO1 and IDO2 expression by RT-PCR. IDO1 activity of Hela cells was blocked by transfection with IDO1-specific siRNA and analysed for tryptophan degradation by RP-HPLC. The impact of d-1MT and l-1MT on IDO activity of Hela cells and protein isolates of human colon cancer were studied.
Results
Human primary gastric, colon and renal cell carcinomas constitutively expressed both, IDO1 and IDO2 mRNA, whereas cancer cells lines had to be induced to by Interferon-gamma (IFN-γ). Treatment of Hela cells with IDO1-specific siRNA resulted in complete abrogation of tryptophan degradation. Only l-1MT, and not d-1MT, was able to block IDO activity in IFN-γ-treated Hela cells as well as in protein isolates of primary human colon cancer.
Conclusions
Although IDO2 is expressed in human tumors, tryptophan degradation is entirely provided by IDO1. Importantly, d-1MT does not inhibit the IDO activity of malignant cells. If ongoing clinical studies show a therapeutic effect of d-1MT, this cannot be attributed to inhibition of IDO in tumor cells.



Similar content being viewed by others
References
Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS, Hunt NH (2007) Characterization of an indoleamine 2, 3-dioxygenase-like protein found in humans and mice. Gene 396(1):203–213
Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G, Weiss HG, Gobel G, Margreiter R, Konigsrainer A, Fuchs D, Amberger A (2006) Prognostic value of indoleamine 2, 3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res 12(4):1144–1151
Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, Mellor AL, Prendergast GC, Munn DH (2007) Inhibition of indoleamine 2, 3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res 67(2):792–801
Ino K, Yoshida N, Kajiyama H, Shibata K, Yamamoto E, Kidokoro K, Takahashi N, Terauchi M, Nawa A, Nomura S, Nagasaka T, Takikawa O, Kikkawa F (2006) Indoleamine 2, 3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer 95(11):1555–1561
Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P (2008) Levo- but not dextro–1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood 111(4):2152–2154
Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC (2007) Prendergast, Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2, 3-dioxygenase inhibitory compound d-1-methyl-tryptophan. Cancer Res 67(15):7082–7087
Munn DH, Mellor AL (2007) Indoleamine 2, 3-dioxygenase and tumor-induced tolerance. J Clin Invest 117(5):1147–1154
Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL (2004) Expression of indoleamine 2, 3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest 114(2):280–290
Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL (1998) Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281(5380):1191–1193
Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, Ishii N, Yanaihara N, Yamada K, Takikawa O, Kawaguchi R, Isonishi S, Tanaka T, Urashima M (2005) Indoleamine 2, 3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res 11(16):6030–6039
Takikawa O, Kuroiwa T, Yamazaki F, Kido R (1988) Mechanism of interferon-gamma action. Characterization of indoleamine 2, 3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J Biol Chem 263(4):2041–2048
Terness P, Chuang JJ, Opelz G (2006) The immunoregulatory role of IDO-producing human dendritic cells revisited. Trends Immunol 27(2):68–73
Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ (2003) Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2, 3-dioxygenase. Nat Med 9(10):1269–1274
Widner B, Fuchs D (2000) Immune activation and degradation of tryptophan. Mod Asp Immunobiol 1:105–108
Zou W (2005) Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 5(4):263–274
Acknowledgments
S.L. was supported by a Fortüne grant of the University of Tübingen (1636-0-0). The authors thank Lynne Yakes for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Löb, S., Königsrainer, A., Zieker, D. et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother 58, 153–157 (2009). https://doi.org/10.1007/s00262-008-0513-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-008-0513-6